-
Effect of Pressure on the Free Ion and Crystal Field Parameters of Sm2+ in BaFBr and SrFBr Hosts
P. Pal, T. Penhouët, V. D'Anna and H. Hagemann
Journal of Luminescence, 134 (2013), p678-685


DOI:10.1016/j.jlumin.2012.07.010 | unige:24061 | Abstract | Article HTML | Article PDF
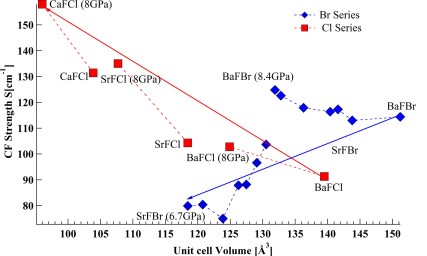
The emission spectra of Sm2+ doped in BaFBr and SrFBr hosts were measured at 10 K from ambient pressure to 8 GPa. The crystal field energy levels determined from the emission spectra were used to extract the free ion parameters (Fk and ζ ) and crystal field parameters (Bqk). The variation of Fk and ζ as a function of pressure was studied systematically and was discussed in relation to the central field and symmetry restricted covalency models. The change of the spin orbit coupling parameter (ζ) with pressure for SrFBr:Sm2+ showed very different behavior than in other matlockite hosts. Moreover the variation of Bqk under pressure was studied. The pressure dependence of the Bqk was described quantitatively using the Superposition Model (SM) with the help of structural parameters as a function of pressure, obtained from periodic DFT calculations. The validity of the SM was tested for Sm2+ in BaFBr and SrFBr. It is shown that this model does not apply to SrFBr, in contrast to other matlockite host materials.